
These substructure features are used to develop QSAR models for prediction of toxicity and various molecular physicochemical properties. 5-membered aromatic ring with 1 heteroatom). This list includes also numerous heterocyclic rings and general structural patterns (i.e. Recently an extended version of the program containing 583 manually curated functionalities encoded as SMARTS was published.
Alkene functional group software#
One of the first software tools to identify FGs was the checkmol program written by Haider that was able to identify about 200 FGs. The fragments are generally strongly overlapping and are generated for all parts of a molecule without considering their potential chemical role. Although such fragment descriptors are very useful, they do not provide description of functional groups. Examples of such descriptors are linear or atom centered fragments, topological torsions, pharmacophoric triplets and many others. In this approach the substructure descriptors are generated by extracting groups of atoms from a molecule using a predefined algorithm. Various substructure features are often used in cheminformatics in connection with machine learning to develop models to predict biological activity or properties of molecules. describing a rule-based definition of chemical classes to classify compounds into classes or the ClassyFire software developed in the Wishart’s group allowing chemists to perform large-scale automated chemical classification based on a structure-based chemical taxonomy consisting of over 4800 categories. An example of this type of publications is work by Bobach et al. The majority of theoretical studies are utilizing FGs as a basis of chemical ontologies, where FGs are “keys” that are used to hierarchically classify molecules into categories. There is, however, surprisingly little attention paid to the study of functional groups from the cheminformatics point of view.
Alkene functional group series#
A well known example is the classical book series “Chemistry of functional groups” describing various classes of organic molecules consisting of over 100 volumes. Numerous scientific papers and books focus on properties and reactivity of various FGs. The study of common FGs forms substantial part of basic organic chemistry curriculum. The concept of functional groups (FGs)-sets of connected atoms that determine properties and reactivity of parent molecule, forms a cornerstone of organic chemistry, medicinal chemistry, toxicity assessment, spectroscopy and, last but not least, also chemical nomenclature. The new method allows the analysis of functional groups in large chemical databases in a way that was not possible using previous approaches.
Alkene functional group full#
The algorithm is relatively simple and full details with examples are provided, therefore implementation in any cheminformatics toolkit should be relatively easy.

ConclusionsĪ new algorithm to identify all functional groups in organic molecules is presented. The procedure is illustrated by extracting functional groups from the bioactive portion of the ChEMBL database, resulting in identification of 3080 unique functional groups. ResultsĪn algorithm to identify functional groups in a molecule based on iterative marching through its atoms is described. The algorithm presented in this article is an attempt to solve this scientific challenge. We are not aware of any program that can identify all functional groups in a molecule automatically.
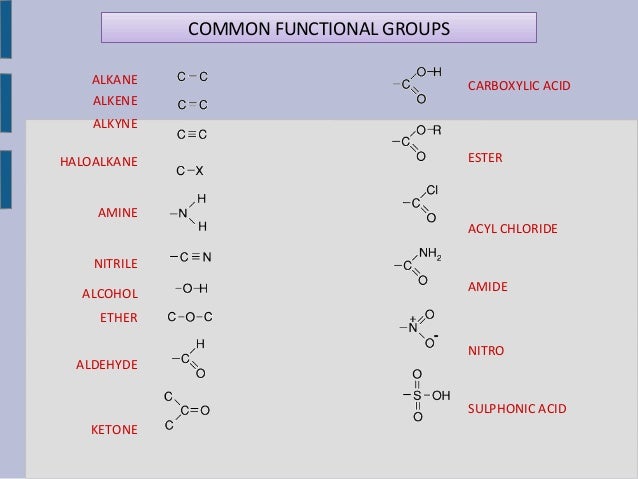
All current software systems to identify functional groups are based on a predefined list of substructures. The reactivity of permanganate ions that are dissolved in such a way is much higher than that of permangante ions in aqueous solution, as they are not solvated.The concept of functional groups forms a basis of organic chemistry, medicinal chemistry, toxicity assessment, spectroscopy and also chemical nomenclature. As a result, potassium ions can be dissolved in an organic solvent, such as benzene, and the negatively charged permangnate ion is, thus, forced to dissolve, as well. 18-crown-6 complexes the potassium ion in its center, while its periphery is non-polar. If the alkene is not water-soluble, potassium permanganate can be made soluble in an organic solvent by the application of the crown ether (a cyclic polyether) 18-crown-6. The intermediate stage of an alkene's oxidative cleavage with permanganate is a 1,2-diol. However, the alkene must contain at least one hydrogen located at the double bond, otherwise only ketones are formed. Alkenes may be converted into carboxylic acid through oxidative cleavage of the double bond with neutral or acid permanganate, for instance. Some of these are further discussed below.Īlcohols and aldehydes may be oxidized into carboxylic acids. There are many possible synthetic pathways that yield carboxylic acids.


 0 kommentar(er)
0 kommentar(er)
